How to Calculate Empirical Formula
Number of empirical formula units in compound 18018 gmol3003 gmol Number of empirical formula units in compound 6 Step 6. Now look for the element with the least number of moles in the compound.

Empirical And Molecular Formula Notes Chemistry Worksheets Teaching Chemistry Chemistry Notes
Steps to determine empirical formula.

. 1 In any empirical formula problem you must first find the mass of the elements in the compound. 32 16 16 64. Assume a 100 g sample of the compound so that the given percentages can be directly converted into grams.
If any of your mole ratios arent whole numbers multiply all numbers by the smallest possible factor that produces whole-number mole ratios for all the elements. Empirical Formula the simplest whole. 4151 x 1 2698 015 Oxygen.
This gives you the ratio between. Since the total mass of the final product was 0378. Calculate the empirical formula.
3 Find the Mole of each atom by doing mass over relative atomic mass you can find the. 2 Write the mass of the atoms. 3692 x 2 16 024 Then we divide the result by the smallest number of moles.
To do this calculate the empirical formula mass and then divide the compound molar mass by the empirical formula mass. Also our online empirical formula calculator considers these equations for finding the simplest positive integer ratio of atoms present in a compound chemistry. Calculate an empirical formula of the compound.
We can find the mole ratios of each element by dividing the individual mole values by the smallest mole value. 015 015 1 Oxygen. This division yields the mole ratios of the elements of the compound.
Steps 1 and 2 are quite hard in this case. Well do some empirical formula calculations and problems in just a second but first lets get some definitions out of the way. Find the molecular formula.
This video goes into detailed steps on how to find the empirical formula of a compound. You have the option of using mass data in grams or percent. So the simplest formula of the compound is CH2O.
Hooray for no more confusionCheck out my NEW complete guide on Empir. From the empirical formula you can work out the molecular formula if you know the relative formula mass Mr of the compound. Find the simplest formula.
Calculate mole ratios of each element. To calculate the empirical formula first determine the relative masses of the elements present. Add up the atomic masses of the atoms in the empirical.
If the molecular formula of the compound is already known we can find its empirical formula just by dividing the number of atoms of each element with a whole number such that we. 024 015 16 Convert. The best thing to do is to first find the number of moles of each of the two.
From the previous step we see that element X and Z both have 333 moles in the compound whereas Y has 666 moles. Then divide all the mole values you calculated in Step 2 by this smallest value. 7 Use the scaling factor computed just above to determine the molecular formula.
1 Write the atoms involved in the calculation. SO 2 times 1 gives SO 2 for. 6 Divide the molecule weight by the EFW 6407 64 1.

Calculating Molecular Formula From Empirical Formula Youtube Great Review Chemistry Lessons Molecular Chemistry Textbook

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular

How To Calculate An Empirical Formula Chemistry Worksheets Chemistry Notes Chemistry Lessons

Calculating Empirical Formula Chemistry Math Formula

The Empirical Formula Of A Compound Is The Simplest Formula That Gives The Correct Relative Numbers Of Teaching Chemistry Chemistry Classroom Chemistry Lessons
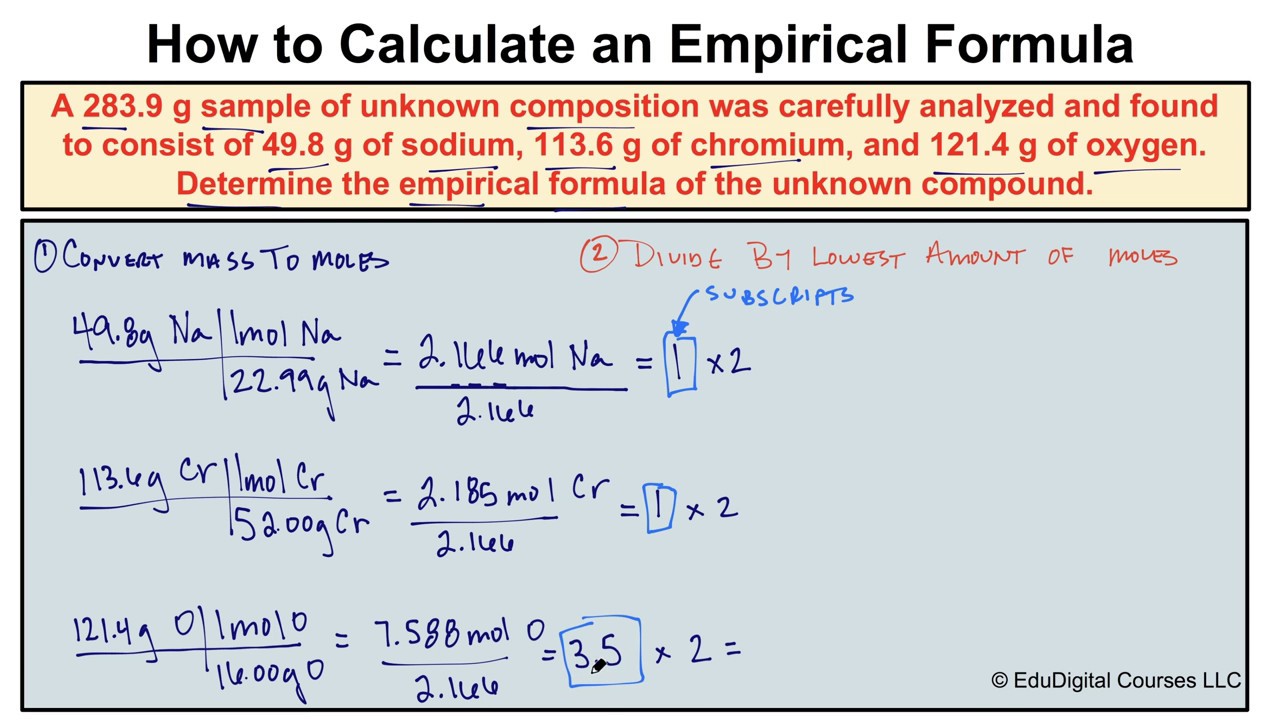
How To Calculate An Empirical Formula Chemistry Worksheets Scientific Method Worksheet Chemistry Notes
0 Response to "How to Calculate Empirical Formula"
Post a Comment